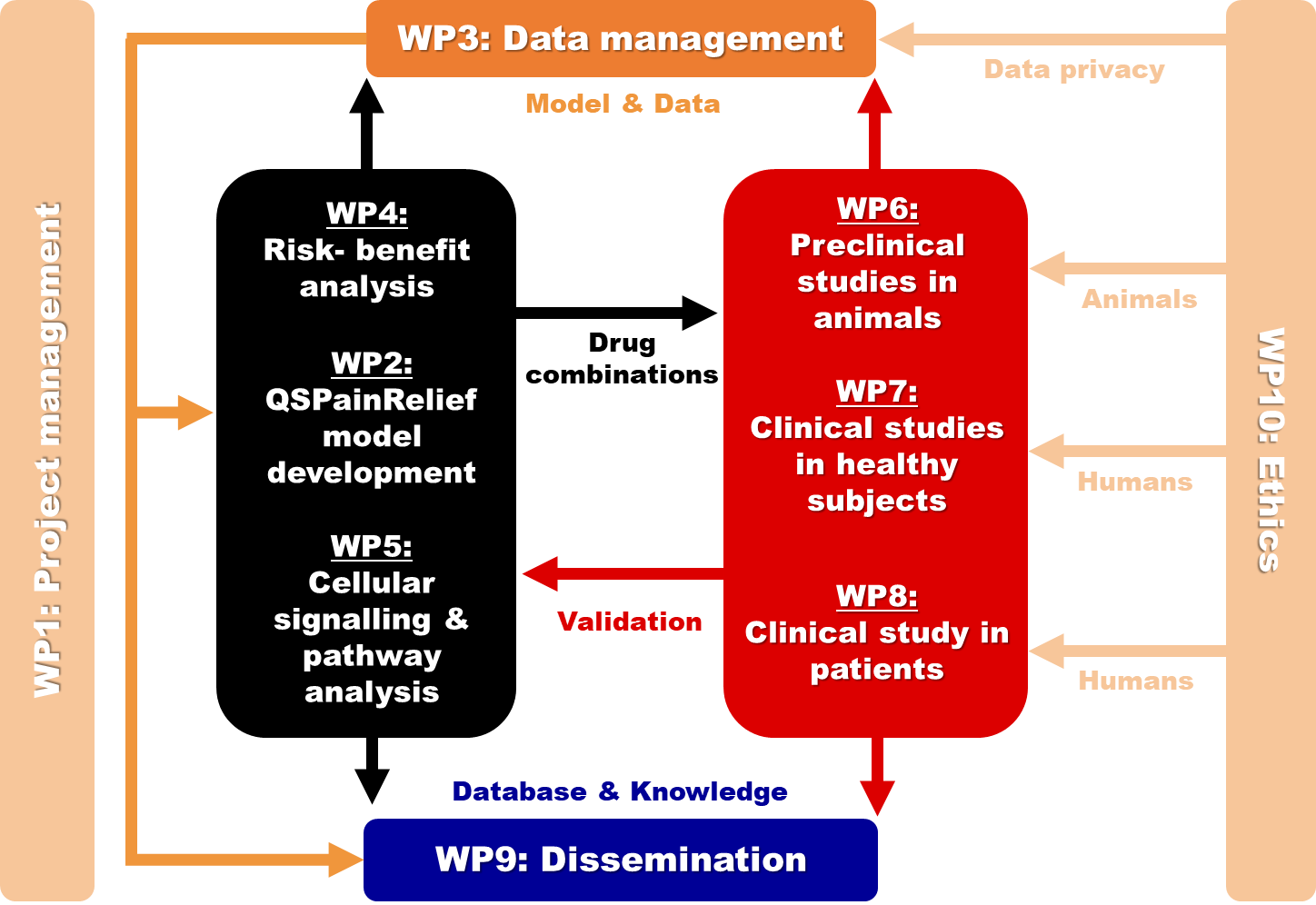
Calibration of the QSPainRelief platform requires patient data on how different drug combinations affect the central processing of nociceptive input, how they affect the CNS networks underlying drug-induced adverse effects, and how these CNS effects translate into real-world clinical therapeutic and adverse effects. Following this calibration step, additional patient data is required to evaluate the ability of the QSPainRelief model platform to prospectively predict CNS and clinical effects of treatment combinations in patients. To that end, we will conduct a clinical study in patients suffering from disabling post-surgical pain to characterize, using non-invasive biomarkers of CNS activity, the effects of different drug combinations on the CNS networks involved in pain perception and two frequent adverse effects that can be readily explored in patients over a short time interval, sedation, and cognitive dysfunction. Furthermore, we will relate these effects of drug combinations on CNS activity with the clinical therapeutic and adverse effects self-reported by the patients. These self-reports, collected up to three months after treatment initiation, will also include an assessment of pain medication misuse, related to drug abuse liability. The objectives of WP8 are:
- To finalize the protocol of the clinical study taking into consideration the human QSPainRelief models for analgesia and adverse effects developed in WP2
- To generate a calibration dataset obtained from the first set of patients that will be used to calibrate the QSPainRelief platform (calibration dataset). The preliminary consensus is to include 60 patients in this dataset
- To generate an evaluation dataset obtained from the second set of patients that will be used to evaluate the QSPainRelief platform (evaluation dataset). The preliminary consensus is to include 120 patients in the evaluation dataset
- To conduct exploratory analyses relating drug-induced CNS effects with real-life treatment effects self-reported by patients